-
Hosting scheme in south-west France lets newcomers sample lifestyle
Households in nine Dordogne communes volunteer under Mes Nouveaux Voisins scheme
-
French boulangeries demand right for staff to work on May 1 so they can open
Artisan bakery owners can work but employees cannot, while certain industrial bakeries are allowed to remain open with workers
-
How many Americans live in Paris - and where else are they choosing in France?
Over a quarter of all US nationals in France live in the capital city
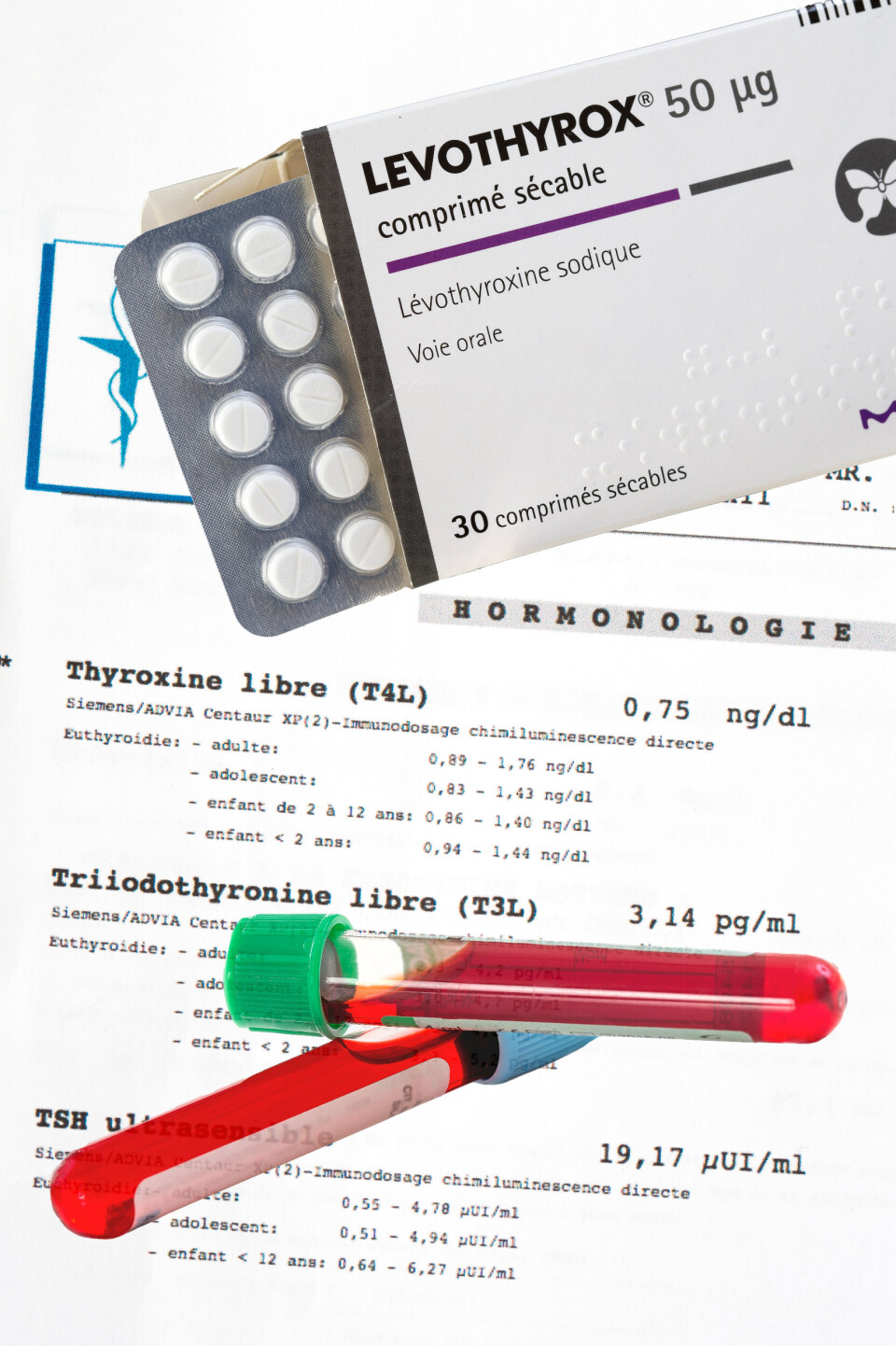
French Levothyrox inquiry now includes manslaughter
The French inquiry into the controversial new formula of thyroid drug Levothyrox has been widened to include the charge of “involuntary homicide”, or manslaughter, after the death of a woman.
Marseille public prosecutor Xavier Tarabeux confirmed that a woman connected to the inquiry had died on November 30 2018.
Until now, the case had focused only on the charge of “endangering life”.
David-Olivier Kaminski, Paris barrister in charge of managing the 200 plaintiff files in the case, said: “This is a real turning point in this case. Some politicians especially had said that this was not a health crisis, and had minimised the complaints of the victims.
“In reality, it appears that the inquiry is now centred on the possibility that the new Levothyrox may have - involuntarily - caused death.”
He added: “This adds a new side to the case; one of gravity, and a real health crisis. Finally, I believe that public authorities must consider this case and the complaints with the utmost seriousness.
“Medicine is supposed to heal, but here, the new formula of Levothyrox was able to unbalance and harm people and their physical conditions. This case shows real injury, and we now discover that it is possible that death has been caused by this medicine.
“The situation is evolving on a deep level, on the gravity of this case. We must trust the judicial proceedings so that, as soon as possible, each person’s responsibilities can be assessed, and those who suffered harm receive compensation.”
Levothyrox is produced by healthcare giant Merck. A new formula of the drug was released in early 2017, and reports began to emerge that it was not effective for some patients in summer that year.
In October 2017, health minister Agnès Buzyn ordered 130,000 boxes of the “old formula” pending an investigation, half of which sold out in two days.
Yet, Merck has always maintained that the “new formula” is essentially medically identical to the old, and that the changes made would not cause the problems claimed by affected patients.
French medicine safety agency ANSM has also confirmed the “good quality” of the drug, despite continued complaints by patients and the case brought by l’Association Française des Malades de la Thyroïde (French association of thyroid patients).
Stay informed:
Sign up to our free weekly e-newsletter
Subscribe to access all our online articles and receive our printed monthly newspaper The Connexion at your home. News analysis, features and practical help for English-speakers in France
























