-
Roadside noise cameras await approval to start issuing fines for loud vehicles in France
The devices known as meduses already exist in several cities but so far have only been ‘instructional’
-
White storks make strong return in France via nest ‘platforms’ and clipped wings
The Ligue pour la Protection des Oiseaux shares the conservation challenges in saving these birds from extinction
-
Hosting scheme in south-west France lets newcomers sample lifestyle
Households in nine Dordogne communes volunteer under Mes Nouveaux Voisins scheme
Almost half of Levothyrox stocks sold in two days
Stocks of an older formulation of the thyroid drug Levothyrox have been selling fast, with almost half of 130 000 boxes that were made available on Monday sold within two days.
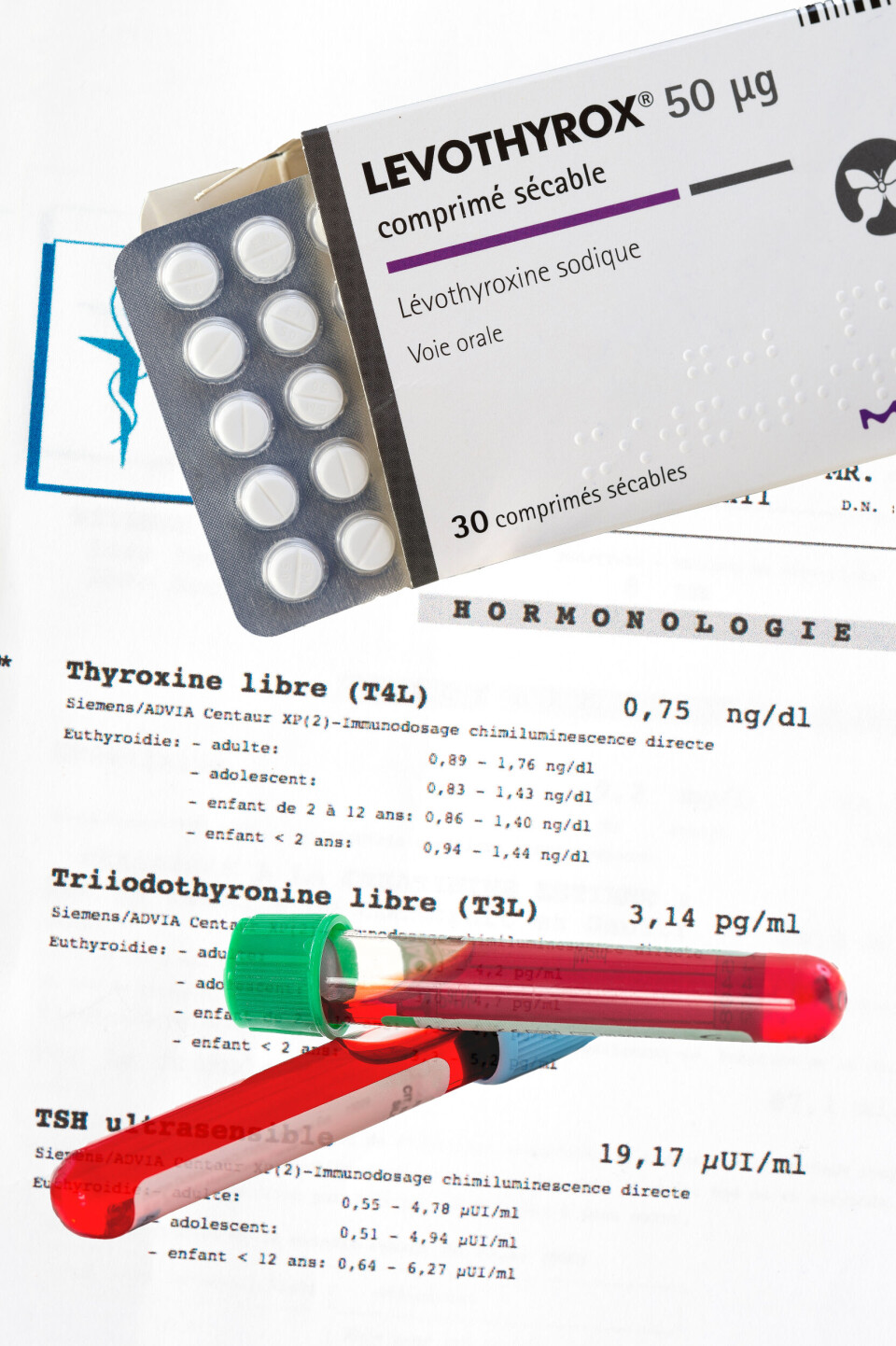
A new formulation of the drug, which is prescribed to over three million users in France for thyroid issues (of which 85% are women), has reportedly been causing serious side-effects including dizziness, headaches, memory loss, extreme fatigue, hair loss, digestive problems, muscle cramps, and depression. These are also side effects of untreated hypothyroid problems, which has led some to suggest that the new drug could be ineffective.
Minister for health, Agnès Buzyn announced in mid-September that the old formulation of the drug would be made available in pharmacies and that priority would be given to those who had suffered bad side effects under the new formulation.
Doctors have been told to prescribe the old formulation only as a last resort to patients who complain of persistent side effects, including cramps, headaches, dizziness and hair loss, that they attribute to the new formulation Levothyrox.
Nearly 60 000 boxes of the old formulation had been sold by the end of Tuesday, the National Agency for Drug Safety (ANSM) said. 70 000 boxes remain, and a further 60 000 should be available by the end of this week, it said. The agency is working with Levothyrox manufacturer Merck on further supplies.
A helpline number was announced in August for patients to call if they were worried about the new drug formulation. An inquiry into complaints surrounding the new drug has also been launched. Over 9 000 complaints about side effects have been recorded to date.
























