-
Roadside noise cameras await approval to start issuing fines for loud vehicles in France
The devices known as meduses already exist in several cities but so far have only been ‘instructional’
-
White storks make strong return in France via nest ‘platforms’ and clipped wings
The Ligue pour la Protection des Oiseaux shares the conservation challenges in saving these birds from extinction
-
Hosting scheme in south-west France lets newcomers sample lifestyle
Households in nine Dordogne communes volunteer under Mes Nouveaux Voisins scheme
French Valneva vaccine maker seeks UK approval despite cancelled order
The UK has cancelled an order of 100 million doses from French company Valneva, which has a base in Scotland

French vaccine company Valneva has said that it is continuing to seek approval for its Covid vaccine in the UK, despite the UK government cancelling an order of 100 million doses from the company earlier this week.
The British government said the company had not met obligations written into the supply deal contract – something the company denies and which it said it could go to court over.
UK health secretary Sajid Javid, meanwhile, said that the vaccine would not have been approved for use in the UK.
“There are commercial reasons that we have cancelled the contract, but what I can [say] is that it was also clear to us that the vaccine in question that the company was developing would not get approval by the MHRA here in the UK,” he said yesterday in parliament (September 14).
Valneva has stated that its phase 3 testing results should be available towards the end of this year and will make up a submission for approval to the UK’s Medicines and Healthcare products Regulatory Agency (MHRA).
The laboratory had said that early trials suggested its vaccine would be more than 80% effective.
Valneva stated in a separate announcement that it had recruited 300 volunteers over the age of 56 in New Zealand for the latest phase 3 testing of the safety of the vaccine.
Unlike the Covid vaccines approved for use in the EU, which use new microbiology techniques to be effective, the Valneva vaccine uses a “traditional” inactivated virus technology to give protection against Covid 19.
“Valneva has commenced rolling submission for conditional approval with the UK’s Medicines and Healthcare Products Regulatory Agency,” the company wrote in a statement on Monday (September 13).
"The contract provides [the British government] with the right to terminate. [The British government] has alleged that the company is in breach of its obligations under the supply agreement, but the company strenuously denies this,” it added.
Valneva’s shares fell by around 40% after news of the cancellation of the €1.4billion deal.
Valneva’s Scottish base
The company, which has its headquarters in Saint-Herblain, near Nantes, is investing in a new factory in Livingstone in Scotland, which was destined to produce most of the vaccines for the British order.
Grants and other help worth €15million were given by the UK for the factory project.
Mr Javid said the British and Scottish governments were working together to assure the future of the factory.
Valneva has not said whether the cancellation will lead to work on the new factory being abandoned.
Valneva’s vaccine in France
French government sources have said that the country is still interested in signing up for the Valneva vaccine and talks have now entered into legal parts of the contract, Reuters reported.
The same sources said that the EU was also still interested in the vaccine.
Earlier this year Valneva said procedures to get EU wide approval for its vaccine were too bureaucratic and slow, and it was instead seeking approval on a country by country basis in the EU, before reversing its position and saying it was still in discussions with Brussels.
The French/Swiss pharmaceutical giant Sanofi is also working on a Covid vaccine based on inactivated virus technology and is on track to have it ready by the end of the year.
Covid vaccines in France
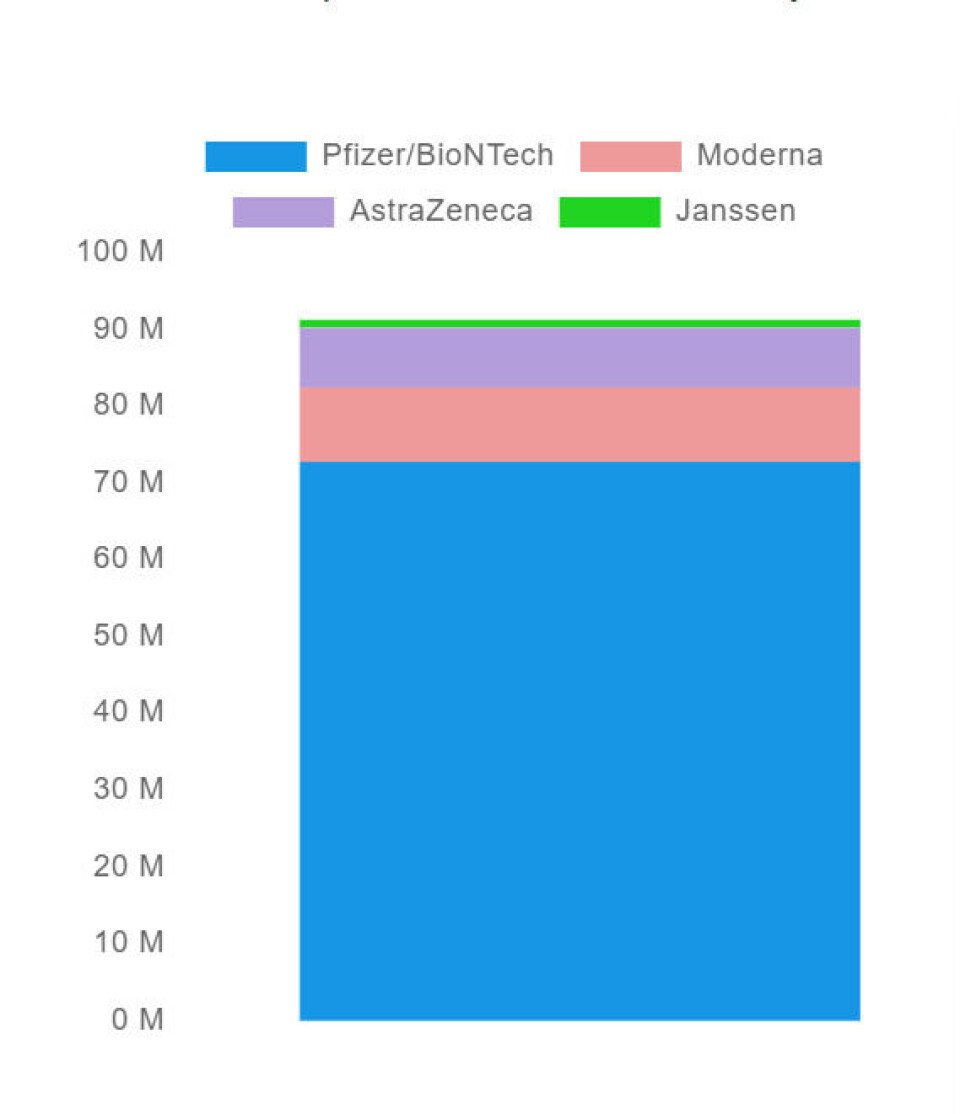
Four Covid vaccines have been approved for marketing use by the EU authority the European Medicines Agency (EMA) and are being administered in France.
They are:
-
Comirnaty (Pfizer-BioNTech)
-
Spikevax (previously known as Moderna)
-
Vaxzevria (previously known as AstraZeneca)
-
COVID-19 Vaccine Janssen (Johnson & Johnson)
The Pfizer vaccine has been the most commonly used in France, with over 70 million doses of it administered. Around 10 million doses of the Moderna and AstraZeneca vaccines have been administered, while only a very small number of doses of the Johnson & Johnson have been administered.
There are a further five vaccines that are under rolling review by the EMA.
This includes the US-made Novavax, German-made CureVac, Russian-made Sputnik V, Chinese-made Sinopharm and French-made Vidprevtyn, from Sanofi, Pasteur Institute and GSK.
The EU currently has an order for doses of Novavax, CureVac and Vidprevtyn.
























