-
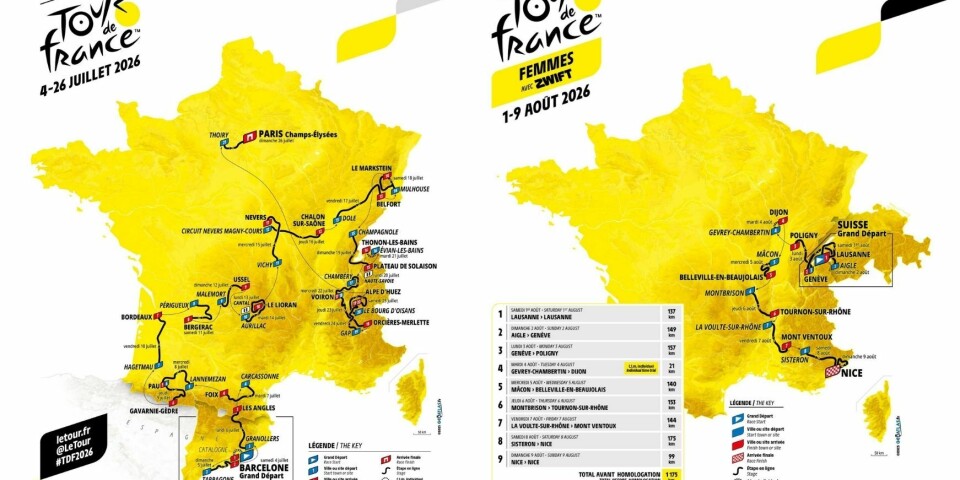
Air France expands US schedule with direct Paris-Las Vegas route
Airline now offers 19 US destinations
-
2025 small business VAT reform definitively cancelled after Senate vote
New 2026 proposals remain on table but likely to be struck out as MP debates get underway
-
Small drop in percentage of French visa applications being declined
Roughly one in every six visa requests refused in 2024
France to offer new treatment for high-risk Covid patients
The new drugs are set to be authorised within days and have been successfully used in the US, including on former President Donald Trump

A Covid monoclonal antibody treatment that could cut hospitalisations for high-risk patients by up to 70% will soon be available in France. What is it, and how does it work? We explain.
Health Minister Olivier Véran yesterday announced that the treatment will soon be available in the country as another way to fight the virus.
He said: “This is a new hope that will help to strengthen our anti-Covid arsenal.”
What is monoclonal antibody treatment?
It is an infusion, which has a neutralising effect on Covid-19, by imitating natural antibodies that the body itself generates to fight the infection.
There are several treatments that come under the title, with names such as Regdanvimab, Bamlanivimab and Etesvimab. It is also known under the name CT-P59.
It is made by laboratories including the South Korean group Celltrion, and American lab Eli Lilly.
How does it work?
Monoclonal antibodies mimic the body’s natural antibodies.
They attach to a given antigen structure - in this case, the SARS-CoV-2 virus, which causes Covid-19 - and attack the virus’ ability to penetrate cells.
Dr Morgane Bomsel, researcher at health unit the CNRS at the Institut Cochin, told newspaper La Dépêche: “It works as a barrier against the spread of the virus in the body, which is compelling because according to initial lab publications, it appears to work just as well on the UK variant [of the virus].”
Professor Yves Coppieters, an epidemiologist, told news service BFMTV: “The idea is to find the most-performing antibodies and produce them in a lab - so they are artificial antibodies - and, through injection, stimulate passive immunity.”
Dr Bomsel said that the treatment was not preventative, and would only work to “limit severe forms of the illness and therefore the number of intensive care admissions”.
The treatment is therefore expected to reduce the number of hospitalisations and intensive care admissions of Covid-19 patients.
Yet, it is expensive. It costs €1,000-€2,000 per dose, so will likely be used only on the patients most at risk of developing a serious form of the disease.
The treatment has previously been used to help treat certain cancers and inflammatory illnesses, and has been repurposed in this case to work against Covid-19.
Is it on the market now?
A treatment called “Regeneron”, by the laboratory Eli Lilly is set to be authorised for use in France in the coming days according to reports, under a temporary use permit.
The treatment named Regdanvimab - which is part of the same family - is still under examination by the European Medicines Agency (EMA), which is looking at initial data from animal studies and clinical trials.
The EMA is evaluating all available data via a process designed to speed up any treatment or vaccines that could significantly improve public health.
If the data shows enough proof that the drug can be authorised for use, it will be validated and checked for the usual standards of safety, efficiency, and quality.
So when will it arrive in France?
On its website, the health ministry yesterday said: “The first treatments based on monoclonal antibodies that have a neutralising effect on the SARS-CoV-2 protein will be available very soon in France.
“This treatment will be added to the therapeutic measures already in place to treat patients that are at risk of developing severe forms of Covid-19.
“Monoclonal antibodies, by stopping the virus from penetrating into cells, and stopping it from replicating, can neutralise the virus at the early stages of infection.”
The health ministry added that national health website sante.fr is set to show a list of establishments that will stock the medicine from March 1, 2021.
Medical safety agency l’Agence de Sécurité du Médicament (ANSM) is aiming to temporarily authorise a treatment from the monoclonal antibody family by the beginning of March, reported news source Le Point.
The government is aiming to acquire more than €170 million-worth of the treatment from the American group Eli Lilly, said newspaper Le Figaro - although this has not yet been confirmed by health authority La Direction générale de la santé.
France is set to be the second country in the EU, after Germany, to introduce the treatment.
Mr Véran said: “Around 83 hospital centres have already received several thousand doses of this treatment, which can begin to be carefully administered in a hospital environment for patients aged 80 and over, who have immune issues.
“Several thousand extra ‘second generation’ doses will be received by mid-March.”
Has the treatment been used successfully against Covid before?
Former President Donald Trump is said to have been administered an antibody-type treatment by American firm Regeneron, when he caught Covid last year.
In his case, the treatment was a combination of two monoclonal antibodies.
Dr Bomsel, at the CNRS, explained: "A mixture of several antibodies is recommended, as in the case of the patient Donald Trump. Once the virus is in the body, it tries to replicate itself, so it is more efficient to inject different antibodies that act in different places.
She said: "It is not a miracle cure. Of course, it is always good to have extra options for treatment, but it is not the solution that will totally eradicate the disease.”
The drugs have been used to successfully treat more than 125,000 patients in the US, with reports saying that it was highly-tolerated by the body, and early studies suggesting that it significantly reduces the chance of death and severe illness from the virus.