-
Pension age reform in France: Most favour return to age 62
A ‘conclave’ of employers' organisations and trade unions is meeting to discuss matter on the the orders of Prime Minister François Bayrou
-
Mystery of jewels found buried under communal wall in Dordogne
The gold rings, pearl brooches and diamond encrusted bracelets were discovered by a local association
-
Try a different way to cross UK-France the Channel - a sailing catamaran ferry
Passengers will be able to help sail the boat once out of the harbour
How safe were Covid vaccines? Side effects studied by French drug body
The vast study assessed the risks from 200,000 reports
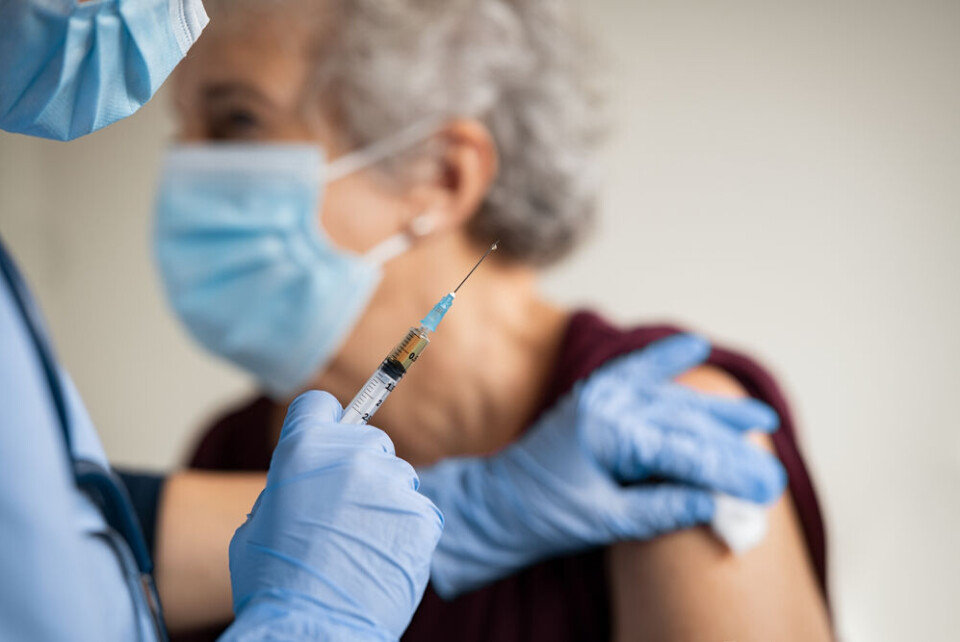
The French drug safety body has presented its work in mapping the extent of side effects after vaccination against Covid-19 in France, three years after the first vaccine was given.
The Réseau français des centres de pharmacovigilance (RFCRPV) looked at 200,000 reports of post-vaccination side effects flagged by health professionals and members of the public over a span of three years, comprising over 157 million vaccinations.
This presented an unprecedented challenge to the drug safety body, largely due to the scale and timeframe of the vaccine rollout.
“In theory there was nothing new about it,” RFCRPV vice-president Dr Aurélie Grandvuillemin told Sciences et Avenir.
“However, in terms of tracking a treatment that was administered in such a short space of time to such a large number of people, it was completely unprecedented.”
The RFCRPV had to determine if the reports of side effects were related to the Covid vaccines while looking for any other effects that may not have been observed during the vaccines’ clinical trials.
Of the 200,000 side effects in the study, 25% (50,000) were classified as “severe”, said national medicines safety agency, l'Agence nationale de sécurité du médicament et des produits de santé (ANSM).
What side effects did it find?
Blood clots - Rare risk
The RFCRPV issued an alert in spring 2021 about the small-but-possible risk of blood clotting events (thrombosis) following a Covid jab.
“We were receiving a number of reports for patients with or without prior risk factors, vaccinated by Pfizer, Moderna or AstraZeneca," said Dr Grandvuillemin. “So something was potentially going on.”
Later investigations showed no statistical excess risk for vaccines using messenger RNA technology (Pfizer/BioNTech, and Moderna). However, a statistical risk (1 per 100,000 people) was found for the AstraZeneca vaccine, particularly in the under-55s.
At the time, the health authorities recorded 30 cases of thrombosis in France, after four million doses of the AstraZeneca vaccine had been given. Nine people died from a thrombosis-related case.
Heart problems - Very low risk
Also in 2021, RFCRPV centres received increased reports of patients with inflammation of the heart (myocarditis) and the membrane around the heart (pericarditis).
They had been vaccinated with messengerRNA (mRNA) vaccines (Pfizer/BioNTech, and Moderna).
However, in July, the ANSM said that a link had been “confirmed”, but that it remained very “rare”, and did not “call into question the benefit/risk ratio of the vaccine”. This meant that the risks of vaccination were considered much lower than the benefits.
The Assurance Maladie came to a similar conclusion in 2022.
It said “that vaccination with Comirnaty (Pfizer-BioNTech) and Spikevax (Moderna) increases the risk of myocarditis and pericarditis in the seven days following vaccination”, especially in men aged under 30. However, the risk was “low, given the high number of doses administered”.
Menstrual disturbances - 20% risk after first dose
Reports of this side effect have been attracting medical interest since the end of 2021.
A recent study by the ANSM showed that there is a 20% risk of heavy menstrual bleeding between one and three months after the injection of a first dose of an mRNA Covid-19 vaccine.
The data shared shows that eight women were affected for every million patients vaccinated.
The European Medicines Agency added the side effect to the official list of risks for mRNA vaccines in October 2022, after the assessment of RFCRPV data. It was also added to the side effects leaflet enclosed within the vaccine packages.
Read more: Covid vaccine disturbs menstruation for some women, finds French study
‘Sometimes we can’t find a link’
"In some cases it is extremely complex and we have to work with almost impossible cases in which we cannot rule either way: a link cannot be established, but cannot be ruled out either”, Dr Grandvuillemin told Sciences et Avenir.
Such cases include:
-
Pain in the extremities, insomnia, malaise, and lymph node problems: Infrequent (between 1 and 10 cases per 1,000 injections).
-
Skin rashes, swelling, or temporary facial paralysis: Rare (between 1 and 10 cases per 10,000 injections).
-
Severe allergic reactions (anaphylaxis): Very rare (less than one case per 100,000 injections).
Nonetheless, the RFCRPV sought to reiterate that vaccination had saved lives.
A World Health Organization (WHO) study from April 2023 estimated that between December 2020 and April 2023, the vaccine had directly prevented just over one million deaths in Europe, with 96% of these people aged over 60.
Vaccination campaign
The latest vaccination campaign in France began in October 2023, and particularly focused on the most at-risk, vulnerable groups.
People can also get their flu vaccination at the same time as their Covid jab.
In its latest report, health authority Santé publique France said that most indicators of Covid-19 are “dropping, or at a weak level”, but indicators of flu are currently rising and are at epidemic levels.
Related articles
Flu vaccination campaign extended due to rise in cases in France
See: big rise in flu cases and hospitalisations in France
Doctors concerned over low take-up of Covid and flu vaccines in France
























