-
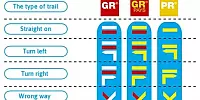
GR, GRP, PR: What do the French hiking signs mean?
What are the coloured symbols on French hiking routes? Who paints them there and why?
-
Miss France: glam - but not sexy
Miss France organiser Geneviève de Fontenay fears she is fighting a losing battle to protect her 'Cinderella dream' from vulgarity
-
Normandy Landings visit for Queen
Queen Elizabeth has confirmed a state visit to France, ending rumours she is handing over duties to Charles
Lab boss says drug did not kill 2,000
Slimming drug has killed “only three people” and not 2,000, says boss of lab that makes Mediator
THE head of the laboratory that makes controversial diet drug Mediator has denied it caused the deaths of between 500 and 2,000 people, saying it has been responsible for only three deaths.
Libération newspaper said Jacques Servier, owner of Laboratoires Servier, had told staff that the figure of 500 deaths was a “nice, round marketing figure”, but the drug could not be blamed for more than three deaths, because the other victims had pre-existing heart problems.
Mediator, which was withdrawn from sale in France in November 2009, has been blamed for causing heart valve problems in patients and the government says the death toll could be as high as 2,000.
Initially prescribed in 1976 for diabetics, the drug was later used as a slimming aid and, despite being under investigation in Europe since 1995, has been taken by more than five million French people.
Although Mr Servier told laboratory staff in a new year message that the drug was not to blame for the large number of deaths, it was reported in Le Figaro this week that a medical study ordered by the laboratory in 2009 had revealed a link between Mediator and heart valve problems.
This week health authorities were urged to ban three other widely prescribed drugs: Buflomédil (sold under brand names such as Fonzylane), which is used as a vasodilator to ease blood pressure problems; nimesulide, usually sold as Nexen, which is an anti-inflammatory; and vinflunine, sold as Javlor, which is used in bladder cancer treatment.
Independent medical journal Préscrire, which first raised the alarm about Mediator, pointed the finger at the three drugs and called for a ban after saying that health authorities had been aware of problems with Buflomédil for more than 20 years and had done nothing other than ban the strongest version.
Health minister Xavier Bertrand, who ordered an inquiry to find out why Mediator had remained on sale, says he is looking into the latest claims about Buflomédil and the other drugs.