-
Britons are the largest foreign community of second-home owners in Nouvelle Aquitaine
See which other departments in the region are popular with British nationals
-
Travellers risk extra costs under new Eurotunnel ticket rule
Some fare options are less flexible and less forgiving of lateness
-
May will be difficult month for train travel in France, warns minister
Two major train unions are threatening to strike and are ‘not willing to negotiate’, he says
Positive signs for Nantes lab's Covid-19 treatment
The antibody treatment aims to stop patients with Covid-19 becoming seriously ill. Its producers say clinical trials could have started much earlier but the government prioritised vaccine development over treatments
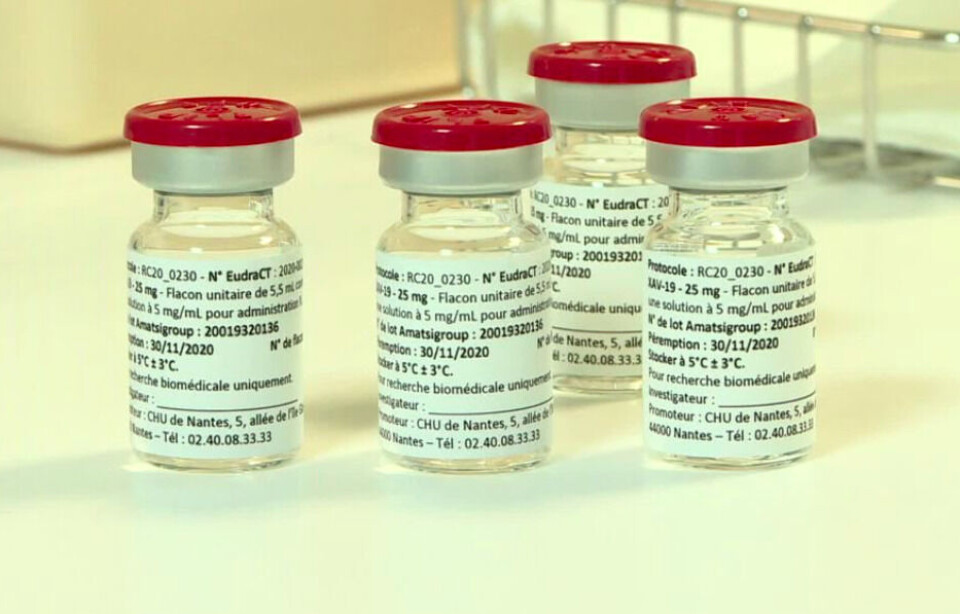
A medical laboratory in Nantes has now completed the first human trials of its promising Covid-19 treatment on 398 patients in 35 French hospitals.
This means it has completed stage two of the clinical trials, and initial (as yet unofficial) findings show the treatment to be safe and not to have serious side effects. Final results including on its effectiveness in the patients will be published by this summer.
The treatment, called Xav-19, is made up of a cocktail of antibodies that attack the virus in multiple ways.
Preclinical trials previously found that with a sufficient concentration, the treatment neutralised 100% of the virus in a test tube.
In a press release earlier this year the laboratory, Xenothera, said the treatment has the effect of “reducing the risk of worsening in patients with moderate forms of the [Covid-19] disease. XAV-19 works by neutralizing the virus, reducing inflammation and limiting the risk of a cytokine storm [a reaction in the immune system which can cause multisystem organ failure].”
The treatment was also found to have a “blocking effect” on all the main variants including those from the UK, South Africa, and Brazil, the laboratory said.
Trials could have begun earlier
Laboratory president Dr Odile Duvaux said the results showing the treatment works had been “great news” but she was disappointed the clinical trials had not begun earlier.
The clinical trials began in September 2020 but the laboratory had been working on its coronavirus treatment long before the start of the health crisis in 2020.
In December 2020, Dr Duvaux told The Connexion: “[France] could have been the first in the world to have an antibody treatment against SARS-CoV- 2. In March 2020, we were ready: we had prepared a treatment for previous coronavirus outbreaks and our technology had been tested on patients.”
Read more: ‘Our Covid-19 treatment was ready but red tape stopped us’
She said testing was delayed because the government had prioritised developing vaccines over treatments in the fight to control the epidemic.
The laboratory plans to publish results from the second stage of clinical trials this summer, and hopes the treatment will be given temporary authorisation by medicines safety agency the l'Agence nationale de sécurité du medicament – the first step towards rolling the medicine out for wider use.
It also hopes to sign pre-orders in France and other European countries in order to start industrial production of the treatment.
Related stories
Will Johnson & Johnson Covid vaccine now be used in France?
French start-up makes exoskeletons to support staff in physical roles
French company launches €100m project to create artificial skin
























