-
Britons are the largest foreign community of second-home owners in Nouvelle Aquitaine
See which other departments in the region are popular with British nationals
-
Travellers risk extra costs under new Eurotunnel ticket rule
Some fare options are less flexible and less forgiving of lateness
-
May will be difficult month for train travel in France, warns minister
Two major train unions are threatening to strike and are ‘not willing to negotiate’, he says
Recap: How will France’s child Covid vaccination programme work?
Children aged five to 11 will soon be offered vaccines in two stages, subject to approval from France’s health authorities
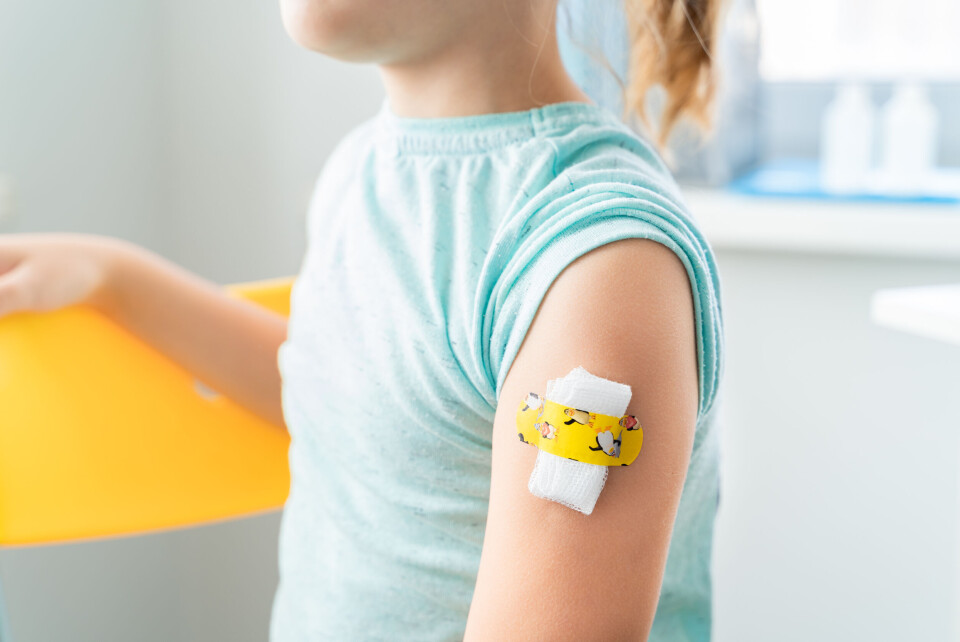
Covid vaccines will be offered to children in France aged five to 11 by the end of the year “if possible”, the government has said.
Children in this age group who are more “at risk” from Covid will be offered the vaccine from December 15, announced Health Minister Olivier Véran and Prime Minister Jean Castex on Monday (December 6).
Read more: France announces six new Covid measures in bid to combat fifth wave
Mr Véran said that: “The first delivery of Pfizer vaccines with a dilution suitable for children will be made on December 13.”
This move has already been approved by France’s health service quality regulator, the Haute Autorité de Santé (HAS), which has recommended that priority be given to children with: “chronic hepatic illnesses, chronic cardiac and respiratory illnesses (including severe asthma requiring continuous treatment), neurological illnesses, immunodeficiency – whether from birth or brought on by medication – obesity, diabetes, haematological malignancies, sickle cell anaemia and Down’s Syndrome.”
This concerns around 360,000 children across the country.
However, the government is still waiting to hear the recommendations of HAS and the Comité consultatif national d’éthique ethics committee with regards to the vaccination of other children.
If this vaccination rollout is approved, the government aims to have “all logistics to start vaccinating children as soon as possible” in place by December 20. It is hoped that all five to 11-year-olds will have been offered their vaccine by the end of the year, although they will not be obliged to receive it.
Vaccination centres would be the first to be able to vaccinate this age group, followed by GPs and pharmacies around December 27.
This second stage of the rollout would concern approximately six million children, who will receive two injections of a vaccine whose dosage is three times smaller than that given to adults, containing 10 micrograms of antigens as opposed to 30. Doses can be given three weeks apart.
Parents or guardians who are unsure whether their child fits into the “at risk” category should contact their doctor.
Only one parent or guardian needs to give their permission for the vaccination of their child.
Why begin vaccinating children now?
The government is particularly eager to vaccinate five to 11-year-olds because Covid case numbers are especially high among people under the age of 19, with an infection rate of more than 530 per 100,000 of the population.
Among six to 10-year-olds, the rate currently exceeds 900 per 100,000 people, more than double the national average of 448.
These elevated infection rates can be attributed in part to the high number of tests that are carried out in schools, but they have nonetheless prompted the government to raise the Covid alert level to 3 (orange) in such educational settings.
This brings with it stricter rules on mask-wearing, inter-class mixing and cleaning regimes.
It is hoped that vaccinating younger children will help to limit community transmission and to avoid the closure of classes as a result of Covid cases.
Jean-Paul Stahl, infectious diseases specialist at the Centre Hospitalier Universitaire de Grenoble, told Franceinfo that for more vulnerable children “there is no debate, they need to be protected” by being vaccinated.
“Where there is room for debate is the question of vaccinating other children. We are going to impose this measure on these children but it is all to protect adults who do not want to get vaccinated. It is a bit annoying,” he added.
What have vaccine trials shown?
Currently the only vaccine which is authorised for use among five to 11-year-olds is Pfizer-BioNTech.
Clinical trials carried out by Pfizer and involving 2,200 children aged five to 11 have shown that its vaccine is 90.7% effective against symptomatic forms of Covid.
Side effects are similar to those experienced in adults, and include pain at the site of the injection, redness and potential fever.
The sociétés savantes de Pédiatrie et de la Société de Pathologie Infectieuse de Langue Française paediatric research organisations have stated, however, that clinical trials “do not allow for the identification of eventual, rare side effects.
“We must wait for data from drug safety studies, which will be available after the administration of several million doses.”
In several other countries around the world, including the US, Italy and Spain, the vaccination of five to 11-year-olds is already permitted.
Alain Fischer, president of the Conseil d’orientation de la stratégie vaccinale, has said that while “the figures we have are really encouraging,” it is necessary to wait for the outcome of the US’ child vaccination campaign to determine definitively whether there could be any undesirable effects.
“We should have these results quite soon and, I hope, have really solid safety data" after that, he added.
Related stories
Coronavirus: Daily updates on the situation in France
Third Covid dose vital, future doses may be needed, French senate told
Lateral flow tests France: Shortages at pharmacies as demand rises
























